C3 Antibody - #DF13224
| Product: | C3 Antibody |
| Catalog: | DF13224 |
| Description: | Rabbit polyclonal antibody to C3 |
| Application: | WB IHC IF/ICC |
| Reactivity: | Human, Mouse, Rat |
| Prediction: | Pig, Bovine, Rabbit, Dog, Chicken, Xenopus |
| Mol.Wt.: | 187kDa; 187kD(Calculated). |
| Uniprot: | P01024 |
| RRID: | AB_2846243 |
Related Downloads
Protocols
Product Info
*The optimal dilutions should be determined by the end user.
*Tips:
WB: For western blot detection of denatured protein samples. IHC: For immunohistochemical detection of paraffin sections (IHC-p) or frozen sections (IHC-f) of tissue samples. IF/ICC: For immunofluorescence detection of cell samples. ELISA(peptide): For ELISA detection of antigenic peptide.
Cite Format: Affinity Biosciences Cat# DF13224, RRID:AB_2846243.
Fold/Unfold
acylation-stimulating protein cleavage product; AHUS5; ARMD9; ASP; C3 and PZP-like alpha-2-macroglobulin domain-containing protein 1; C3; c3 complement; C3a anaphylatoxin; C3a; C3b; CO3_HUMAN; Complement C3; Complement C3c alpha' chain fragment 2; Complement C3c; Complement component 3; Complement component C3; Complement component C3a; Complement component C3b; Complement factor 3; CPAMD1; Prepro C3;
Immunogens
Plasma. The acylation stimulating protein (ASP) is expressed in adipocytes and released into the plasma during both the fasting and postprandial periods.
- P01024 CO3_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MGPTSGPSLLLLLLTHLPLALGSPMYSIITPNILRLESEETMVLEAHDAQGDVPVTVTVHDFPGKKLVLSSEKTVLTPATNHMGNVTFTIPANREFKSEKGRNKFVTVQATFGTQVVEKVVLVSLQSGYLFIQTDKTIYTPGSTVLYRIFTVNHKLLPVGRTVMVNIENPEGIPVKQDSLSSQNQLGVLPLSWDIPELVNMGQWKIRAYYENSPQQVFSTEFEVKEYVLPSFEVIVEPTEKFYYIYNEKGLEVTITARFLYGKKVEGTAFVIFGIQDGEQRISLPESLKRIPIEDGSGEVVLSRKVLLDGVQNPRAEDLVGKSLYVSATVILHSGSDMVQAERSGIPIVTSPYQIHFTKTPKYFKPGMPFDLMVFVTNPDGSPAYRVPVAVQGEDTVQSLTQGDGVAKLSINTHPSQKPLSITVRTKKQELSEAEQATRTMQALPYSTVGNSNNYLHLSVLRTELRPGETLNVNFLLRMDRAHEAKIRYYTYLIMNKGRLLKAGRQVREPGQDLVVLPLSITTDFIPSFRLVAYYTLIGASGQREVVADSVWVDVKDSCVGSLVVKSGQSEDRQPVPGQQMTLKIEGDHGARVVLVAVDKGVFVLNKKNKLTQSKIWDVVEKADIGCTPGSGKDYAGVFSDAGLTFTSSSGQQTAQRAELQCPQPAARRRRSVQLTEKRMDKVGKYPKELRKCCEDGMRENPMRFSCQRRTRFISLGEACKKVFLDCCNYITELRRQHARASHLGLARSNLDEDIIAEENIVSRSEFPESWLWNVEDLKEPPKNGISTKLMNIFLKDSITTWEILAVSMSDKKGICVADPFEVTVMQDFFIDLRLPYSVVRNEQVEIRAVLYNYRQNQELKVRVELLHNPAFCSLATTKRRHQQTVTIPPKSSLSVPYVIVPLKTGLQEVEVKAAVYHHFISDGVRKSLKVVPEGIRMNKTVAVRTLDPERLGREGVQKEDIPPADLSDQVPDTESETRILLQGTPVAQMTEDAVDAERLKHLIVTPSGCGEQNMIGMTPTVIAVHYLDETEQWEKFGLEKRQGALELIKKGYTQQLAFRQPSSAFAAFVKRAPSTWLTAYVVKVFSLAVNLIAIDSQVLCGAVKWLILEKQKPDGVFQEDAPVIHQEMIGGLRNNNEKDMALTAFVLISLQEAKDICEEQVNSLPGSITKAGDFLEANYMNLQRSYTVAIAGYALAQMGRLKGPLLNKFLTTAKDKNRWEDPGKQLYNVEATSYALLALLQLKDFDFVPPVVRWLNEQRYYGGGYGSTQATFMVFQALAQYQKDAPDHQELNLDVSLQLPSRSSKITHRIHWESASLLRSEETKENEGFTVTAEGKGQGTLSVVTMYHAKAKDQLTCNKFDLKVTIKPAPETEKRPQDAKNTMILEICTRYRGDQDATMSILDISMMTGFAPDTDDLKQLANGVDRYISKYELDKAFSDRNTLIIYLDKVSHSEDDCLAFKVHQYFNVELIQPGAVKVYAYYNLEESCTRFYHPEKEDGKLNKLCRDELCRCAEENCFIQKSDDKVTLEERLDKACEPGVDYVYKTRLVKVQLSNDFDEYIMAIEQTIKSGSDEVQVGQQRTFISPIKCREALKLEEKKHYLMWGLSSDFWGEKPNLSYIIGKDTWVEHWPEEDECQDEENQKQCQDLGAFTESMVVFGCPN
Predictions
Score>80(red) has high confidence and is suggested to be used for WB detection. *The prediction model is mainly based on the alignment of immunogen sequences, the results are for reference only, not as the basis of quality assurance.
High(score>80) Medium(80>score>50) Low(score<50) No confidence
PTMs - P01024 As Substrate
| Site | PTM Type | Enzyme | Source |
|---|---|---|---|
| S38 | Phosphorylation | Uniprot | |
| S70 | Phosphorylation | Uniprot | |
| N85 | N-Glycosylation | Uniprot | |
| S283 | Phosphorylation | Uniprot | |
| S287 | Phosphorylation | Uniprot | |
| K289 | Ubiquitination | Uniprot | |
| S297 | Phosphorylation | Uniprot | |
| S303 | Phosphorylation | Uniprot | |
| K305 | Ubiquitination | Uniprot | |
| S672 | Phosphorylation | Uniprot | |
| T676 | Phosphorylation | Uniprot | |
| Y686 | Phosphorylation | Uniprot | |
| S742 | Phosphorylation | Uniprot | |
| T885 | Phosphorylation | Uniprot | |
| S922 | Phosphorylation | Uniprot | |
| S928 | Phosphorylation | Uniprot | |
| N939 | N-Glycosylation | Uniprot | |
| S968 | Phosphorylation | Uniprot | |
| T1031 | Phosphorylation | Uniprot | |
| Y1194 | Phosphorylation | Uniprot | |
| S1315 | Phosphorylation | Uniprot | |
| S1321 | Phosphorylation | Uniprot | |
| T1341 | Phosphorylation | Uniprot | |
| S1343 | Phosphorylation | Uniprot | |
| Y1480 | Phosphorylation | Uniprot | |
| S1571 | Phosphorylation | Uniprot | |
| S1573 | Phosphorylation | Uniprot | |
| S1586 | Phosphorylation | Uniprot | |
| N1617 | N-Glycosylation | Uniprot |
Research Backgrounds
C3 plays a central role in the activation of the complement system. Its processing by C3 convertase is the central reaction in both classical and alternative complement pathways. After activation C3b can bind covalently, via its reactive thioester, to cell surface carbohydrates or immune aggregates.
Derived from proteolytic degradation of complement C3, C3a anaphylatoxin is a mediator of local inflammatory process. In chronic inflammation, acts as a chemoattractant for neutrophils (By similarity). It induces the contraction of smooth muscle, increases vascular permeability and causes histamine release from mast cells and basophilic leukocytes.
Acts as a chemoattractant for neutrophils in chronic inflammation.
adipogenic hormone that stimulates triglyceride (TG) synthesis and glucose transport in adipocytes, regulating fat storage and playing a role in postprandial TG clearance. Appears to stimulate TG synthesis via activation of the PLC, MAPK and AKT signaling pathways. Ligand for C5AR2. Promotes the phosphorylation, ARRB2-mediated internalization and recycling of C5AR2.
C3b is rapidly split in two positions by factor I and a cofactor to form iC3b (inactivated C3b) and C3f which is released. Then iC3b is slowly cleaved (possibly by factor I) to form C3c (beta chain + alpha' chain fragment 1 + alpha' chain fragment 2), C3dg and C3f. Other proteases produce other fragments such as C3d or C3g. C3a is further processed by carboxypeptidases to release the C-terminal arginine residue generating the acylation stimulating protein (ASP). Levels of ASP are increased in adipocytes in the postprandial period and by insulin and dietary chylomicrons.
(Microbial infection) C3 is cleaved by Staphylococcus aureus aureolysin; this cleavage renders C3a and C3b inactive. C3b is rapidly degraded by host factors CFH and CFI preventing its deposition on the bacterial surface while C3a is further inactivated by aureolysin.
Phosphorylated by FAM20C in the extracellular medium.
Secreted.
Plasma. The acylation stimulating protein (ASP) is expressed in adipocytes and released into the plasma during both the fasting and postprandial periods.
C3 precursor is first processed by the removal of 4 Arg residues, forming two chains, beta and alpha, linked by a disulfide bond. C3 convertase activates C3 by cleaving the alpha chain, releasing C3a anaphylatoxin and generating C3b (beta chain + alpha' chain). Forms the pro-C3-convertase enzyme complex by interacting with Complement factor B Bb fragment (Bb), which is then stabilized by binding CFP, allowing the complex to become active. The interaction with Bb is dependent on Mg2+. C3b interacts with CR1 (via Sushi 8 and Sushi 9 domains). C3b interacts with CFH. C3d interacts with CFH. C3dg interacts with CR2 (via the N-terminal Sushi domains 1 and 2). During pregnancy, C3dg exists as a complex (probably a 2:2:2 heterohexamer) with AGT and the proform of PRG2. Interacts with VSIG4. Interacts (both C3a and ASP) with C5AR2; the interaction occurs with higher affinity for ASP, enhancing the phosphorylation and activation of C5AR2, recruitment of ARRB2 to the cell surface and endocytosis of GRP77.
(Microbial infection) C3b interacts with herpes simplex virus 1 (HHV-1) and herpes simplex virus 2 (HHV-2) envelope glycoprotein C; this interaction inhibits the activation of the complement system.
(Microbial infection) Interacts with Staphylococcus aureus immunoglobulin-binding protein Sbi; this interaction prevents the association between C3dg and CR2.
(Microbial infection) Interacts with Staphylococcus aureus protein Fib.
Research Fields
· Cellular Processes > Transport and catabolism > Phagosome. (View pathway)
· Human Diseases > Infectious diseases: Bacterial > Pertussis.
· Human Diseases > Infectious diseases: Bacterial > Legionellosis.
· Human Diseases > Infectious diseases: Parasitic > Leishmaniasis.
· Human Diseases > Infectious diseases: Parasitic > Chagas disease (American trypanosomiasis).
· Human Diseases > Infectious diseases: Bacterial > Staphylococcus aureus infection.
· Human Diseases > Infectious diseases: Bacterial > Tuberculosis.
· Human Diseases > Infectious diseases: Viral > Herpes simplex infection.
· Human Diseases > Cancers: Overview > Viral carcinogenesis.
· Human Diseases > Immune diseases > Systemic lupus erythematosus.
· Organismal Systems > Immune system > Complement and coagulation cascades. (View pathway)
References
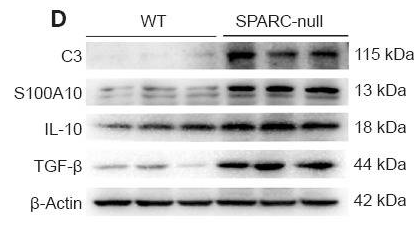
Application: WB Species: Mouse Sample:
Application: IF/ICC Species: Mouse Sample:
Application: WB Species: Rat Sample: spinal cord
Restrictive clause
Affinity Biosciences tests all products strictly. Citations are provided as a resource for additional applications that have not been validated by Affinity Biosciences. Please choose the appropriate format for each application and consult Materials and Methods sections for additional details about the use of any product in these publications.
For Research Use Only.
Not for use in diagnostic or therapeutic procedures. Not for resale. Not for distribution without written consent. Affinity Biosciences will not be held responsible for patent infringement or other violations that may occur with the use of our products. Affinity Biosciences, Affinity Biosciences Logo and all other trademarks are the property of Affinity Biosciences LTD.