ACSL4/FACL4 Antibody - #DF12141
| Product: | ACSL4/FACL4 Antibody |
| Catalog: | DF12141 |
| Description: | Rabbit polyclonal antibody to ACSL4/FACL4 |
| Application: | WB IHC IF/ICC |
| Reactivity: | Human, Mouse, Rat |
| Prediction: | Pig, Bovine, Horse, Sheep, Rabbit, Dog, Chicken, Xenopus |
| Mol.Wt.: | 79 kDa,74 kDa; 79kD(Calculated). |
| Uniprot: | O60488 |
| RRID: | AB_2844946 |
Related Downloads
Protocols
Product Info
*The optimal dilutions should be determined by the end user.
*Tips:
WB: For western blot detection of denatured protein samples. IHC: For immunohistochemical detection of paraffin sections (IHC-p) or frozen sections (IHC-f) of tissue samples. IF/ICC: For immunofluorescence detection of cell samples. ELISA(peptide): For ELISA detection of antigenic peptide.
Cite Format: Affinity Biosciences Cat# DF12141, RRID:AB_2844946.
Fold/Unfold
ACS 4; ACS4; ACSL 4; Acsl4; ACSL4_HUMAN; acyl CoA synthetase 4; Acyl CoA synthetase long chain family member 4; FACL 4; FACL4; Fatty acid Coenzyme A ligase; fatty acid Coenzyme A ligase long-chain 4; LACS 4; LACS4; Lignoceroyl CoA synthase; Long chain 4; long chain acyl CoA synthetase 4; long chain fatty acid CoA ligase 4; long chain fatty acid Coenzyme A ligase 4; Long-chain acyl-CoA synthetase 4; Long-chain-fatty-acid--CoA ligase 4; MRX63; MRX68;
Immunogens
- O60488 ACSL4_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MKLKLNVLTIILLPVHLLITIYSALIFIPWYFLTNAKKKNAMAKRIKAKPTSDKPGSPYRSVTHFDSLAVIDIPGADTLDKLFDHAVSKFGKKDSLGTREILSEENEMQPNGKVFKKLILGNYKWMNYLEVNRRVNNFGSGLTALGLKPKNTIAIFCETRAEWMIAAQTCFKYNFPLVTLYATLGKEAVVHGLNESEASYLITSVELLESKLKTALLDISCVKHIIYVDNKAINKAEYPEGFEIHSMQSVEELGSNPENLGIPPSRPTPSDMAIVMYTSGSTGRPKGVMMHHSNLIAGMTGQCERIPGLGPKDTYIGYLPLAHVLELTAEISCFTYGCRIGYSSPLTLSDQSSKIKKGSKGDCTVLKPTLMAAVPEIMDRIYKNVMSKVQEMNYIQKTLFKIGYDYKLEQIKKGYDAPLCNLLLFKKVKALLGGNVRMMLSGGAPLSPQTHRFMNVCFCCPIGQGYGLTESCGAGTVTEVTDYTTGRVGAPLICCEIKLKDWQEGGYTINDKPNPRGEIVIGGQNISMGYFKNEEKTAEDYSVDENGQRWFCTGDIGEFHPDGCLQIIDRKKDLVKLQAGEYVSLGKVEAALKNCPLIDNICAFAKSDQSYVISFVVPNQKRLTLLAQQKGVEGTWVDICNNPAMEAEILKEIREAANAMKLERFEIPIKVRLSPEPWTPETGLVTDAFKLKRKELRNHYLKDIERMYGGK
Predictions
Score>80(red) has high confidence and is suggested to be used for WB detection. *The prediction model is mainly based on the alignment of immunogen sequences, the results are for reference only, not as the basis of quality assurance.
High(score>80) Medium(80>score>50) Low(score<50) No confidence
PTMs - O60488 As Substrate
| Site | PTM Type | Enzyme | Source |
|---|---|---|---|
| S23 | Phosphorylation | Uniprot | |
| Y31 | Phosphorylation | Uniprot | |
| T34 | Phosphorylation | Uniprot | |
| K47 | Ubiquitination | Uniprot | |
| K49 | Ubiquitination | Uniprot | |
| K54 | Ubiquitination | Uniprot | |
| S57 | Phosphorylation | Uniprot | |
| T63 | Phosphorylation | Uniprot | |
| K89 | Acetylation | Uniprot | |
| K89 | Ubiquitination | Uniprot | |
| K92 | Acetylation | Uniprot | |
| S95 | Phosphorylation | Uniprot | |
| K113 | Ubiquitination | Uniprot | |
| K117 | Ubiquitination | Uniprot | |
| S140 | Phosphorylation | Uniprot | |
| T143 | Phosphorylation | Uniprot | |
| K148 | Ubiquitination | Uniprot | |
| K150 | Ubiquitination | Uniprot | |
| K211 | Ubiquitination | Uniprot | |
| C221 | S-Nitrosylation | Uniprot | |
| K223 | Ubiquitination | Uniprot | |
| Y227 | Phosphorylation | Uniprot | |
| K231 | Ubiquitination | Uniprot | |
| K312 | Ubiquitination | Uniprot | |
| S352 | Phosphorylation | Uniprot | |
| S353 | Phosphorylation | Uniprot | |
| K354 | Ubiquitination | Uniprot | |
| K356 | Ubiquitination | Uniprot | |
| K360 | Ubiquitination | Uniprot | |
| K367 | Ubiquitination | Uniprot | |
| K383 | Ubiquitination | Uniprot | |
| K388 | Ubiquitination | Uniprot | |
| K397 | Acetylation | Uniprot | |
| K397 | Ubiquitination | Uniprot | |
| K401 | Acetylation | Uniprot | |
| K401 | Ubiquitination | Uniprot | |
| Y404 | Phosphorylation | Uniprot | |
| K407 | Ubiquitination | Uniprot | |
| K413 | Ubiquitination | Uniprot | |
| Y415 | Phosphorylation | Uniprot | |
| K426 | Ubiquitination | Uniprot | |
| S447 | Phosphorylation | Uniprot | |
| Y483 | Phosphorylation | Uniprot | |
| T485 | Phosphorylation | Uniprot | |
| K498 | Ubiquitination | Uniprot | |
| K500 | Ubiquitination | Uniprot | |
| T508 | Phosphorylation | Uniprot | |
| K512 | Ubiquitination | Uniprot | |
| K536 | Ubiquitination | Uniprot | |
| Y541 | Phosphorylation | Uniprot | |
| Y582 | Phosphorylation | Uniprot | |
| S584 | Phosphorylation | Uniprot | |
| K587 | Ubiquitination | Uniprot | |
| K593 | Ubiquitination | Uniprot | |
| S607 | Phosphorylation | Uniprot | |
| K621 | Ubiquitination | Uniprot | |
| K651 | Ubiquitination | Uniprot | |
| K661 | Ubiquitination | Uniprot | |
| K670 | Ubiquitination | Uniprot | |
| S674 | Phosphorylation | Uniprot | |
| T679 | Phosphorylation | Uniprot | |
| T682 | Phosphorylation | Uniprot | |
| T686 | Phosphorylation | Uniprot | |
| K690 | Acetylation | Uniprot | |
| K690 | Ubiquitination | Uniprot | |
| K702 | Ubiquitination | Uniprot |
Research Backgrounds
Catalyzes the conversion of long-chain fatty acids to their active form acyl-CoA for both synthesis of cellular lipids, and degradation via beta-oxidation. Preferentially activates arachidonate and eicosapentaenoate as substrates. Preferentially activates 8,9-EET > 14,15-EET > 5,6-EET > 11,12-EET. Modulates glucose-stimulated insulin secretion by regulating the levels of unesterified EETs (By similarity). Modulates prostaglandin E2 secretion.
Mitochondrion outer membrane>Single-pass type III membrane protein. Peroxisome membrane>Single-pass type III membrane protein. Microsome membrane>Single-pass type III membrane protein. Endoplasmic reticulum membrane>Single-pass type III membrane protein. Cell membrane.
Belongs to the ATP-dependent AMP-binding enzyme family.
Research Fields
· Cellular Processes > Transport and catabolism > Peroxisome. (View pathway)
· Cellular Processes > Cell growth and death > Ferroptosis. (View pathway)
· Metabolism > Lipid metabolism > Fatty acid biosynthesis.
· Metabolism > Lipid metabolism > Fatty acid degradation.
· Metabolism > Global and overview maps > Metabolic pathways.
· Metabolism > Global and overview maps > Fatty acid metabolism.
· Organismal Systems > Endocrine system > PPAR signaling pathway.
· Organismal Systems > Endocrine system > Adipocytokine signaling pathway.
References
Application: WB Species: Rat Sample: IEC-6 cells and Caco-2 cells
Application: IHC Species: Mouse Sample: colonic tissues
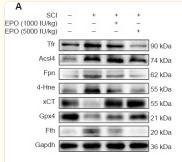
Application: WB Species: Rat Sample: spinal cord
Application: WB Species: Rat Sample:
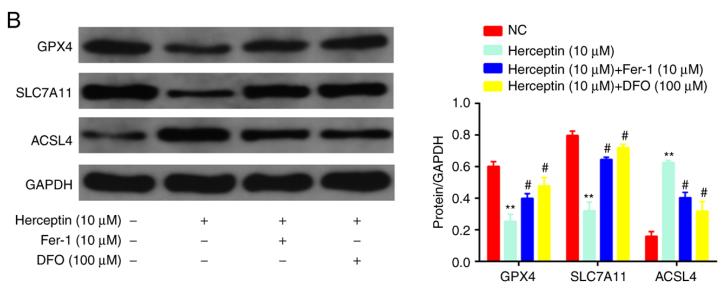
Application: WB Species: Rat Sample: H9c2 cells
Application: WB Species: rat Sample: H9c2 cells
Restrictive clause
Affinity Biosciences tests all products strictly. Citations are provided as a resource for additional applications that have not been validated by Affinity Biosciences. Please choose the appropriate format for each application and consult Materials and Methods sections for additional details about the use of any product in these publications.
For Research Use Only.
Not for use in diagnostic or therapeutic procedures. Not for resale. Not for distribution without written consent. Affinity Biosciences will not be held responsible for patent infringement or other violations that may occur with the use of our products. Affinity Biosciences, Affinity Biosciences Logo and all other trademarks are the property of Affinity Biosciences LTD.