Smad1/5/9 Antibody - #AF0614
| Product: | Smad1/5/9 Antibody |
| Catalog: | AF0614 |
| Description: | Rabbit polyclonal antibody to Smad1/5/9 |
| Application: | WB IHC IF/ICC |
| Reactivity: | Human, Mouse, Rat |
| Prediction: | Pig, Zebrafish, Bovine, Sheep, Rabbit, Dog, Chicken, Xenopus |
| Mol.Wt.: | 56kDa; 52kD(Calculated). |
| Uniprot: | Q15797 | Q99717 | O15198 |
| RRID: | AB_2834266 |
Related Downloads
Protocols
Product Info
*The optimal dilutions should be determined by the end user.
*Tips:
WB: For western blot detection of denatured protein samples. IHC: For immunohistochemical detection of paraffin sections (IHC-p) or frozen sections (IHC-f) of tissue samples. IF/ICC: For immunofluorescence detection of cell samples. ELISA(peptide): For ELISA detection of antigenic peptide.
Cite Format: Affinity Biosciences Cat# AF0614, RRID:AB_2834266.
Fold/Unfold
BSP-1; BSP1; HsMAD1; JV4-1; JV41; MAD homolog 1; MAD mothers against decapentaplegic homolog 1; Mad related protein 1; Mad-related protein 1; MADH1; MADR1; Mothers against decapentaplegic homolog 1; Mothers against DPP homolog 1; SMA- AND MAD-RELATED PROTEIN 1; SMAD 1; SMAD family member 1; SMAD mothers against DPP homolog 1; Smad1; SMAD1_HUMAN; TGF beta signaling protein 1; Transforming growth factor-beta-signaling protein 1; DKFZp781C1895; DKFZp781O1323; Dwfc; hSmad5; JV5 1; JV5-1; MAD homolog 5; MAD, mothers against decapentaplegic homolog 5; MADH 5; MADH5; Mothers against decapentaplegic homolog 5; mothers against decapentaplegic, drosophila, homolog of, 5; Mothers against DPP homolog 5; MusMLP; SMA and MAD related protein 5; SMAD 5; SMAD family member 5; SMAD, mothers against DPP homolog 5; Smad5; SMAD5_HUMAN; MAD homolog 9; Madh6; Mothers against decapentaplegic; Mothers against decapentaplegic homolog 9; Mothers against DPP homolog 9; SMAD 9; SMAD family member 9; SMAD, mothers against DPP homolog 9 (Drosophila); SMAD8A; SMAD8B; Smad9; SMAD9_HUMAN;
Immunogens
Ubiquitous. Highest expression seen in the heart and skeletal muscle.
Q99717 SMAD5_HUMAN:Ubiquitous.
O15198 SMAD9_HUMAN:Expressed in heart, brain, placenta, lung, skeletal muscle, prostate, testis, ovary and small intestine. Also expressed in fetal brain, lung and kidney.
- Q15797 SMAD1_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MNVTSLFSFTSPAVKRLLGWKQGDEEEKWAEKAVDALVKKLKKKKGAMEELEKALSCPGQPSNCVTIPRSLDGRLQVSHRKGLPHVIYCRVWRWPDLQSHHELKPLECCEFPFGSKQKEVCINPYHYKRVESPVLPPVLVPRHSEYNPQHSLLAQFRNLGQNEPHMPLNATFPDSFQQPNSHPFPHSPNSSYPNSPGSSSSTYPHSPTSSDPGSPFQMPADTPPPAYLPPEDPMTQDGSQPMDTNMMAPPLPSEINRGDVQAVAYEEPKHWCSIVYYELNNRVGEAFHASSTSVLVDGFTDPSNNKNRFCLGLLSNVNRNSTIENTRRHIGKGVHLYYVGGEVYAECLSDSSIFVQSRNCNYHHGFHPTTVCKIPSGCSLKIFNNQEFAQLLAQSVNHGFETVYELTKMCTIRMSFVKGWGAEYHRQDVTSTPCWIEIHLHGPLQWLDKVLTQMGSPHNPISSVS
- Q99717 SMAD5_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MTSMASLFSFTSPAVKRLLGWKQGDEEEKWAEKAVDALVKKLKKKKGAMEELEKALSSPGQPSKCVTIPRSLDGRLQVSHRKGLPHVIYCRVWRWPDLQSHHELKPLDICEFPFGSKQKEVCINPYHYKRVESPVLPPVLVPRHNEFNPQHSLLVQFRNLSHNEPHMPQNATFPDSFHQPNNTPFPLSPNSPYPPSPASSTYPNSPASSGPGSPFQLPADTPPPAYMPPDDQMGQDNSQPMDTSNNMIPQIMPSISSRDVQPVAYEEPKHWCSIVYYELNNRVGEAFHASSTSVLVDGFTDPSNNKSRFCLGLLSNVNRNSTIENTRRHIGKGVHLYYVGGEVYAECLSDSSIFVQSRNCNFHHGFHPTTVCKIPSSCSLKIFNNQEFAQLLAQSVNHGFEAVYELTKMCTIRMSFVKGWGAEYHRQDVTSTPCWIEIHLHGPLQWLDKVLTQMGSPLNPISSVS
- O15198 SMAD9_HUMAN:
- Protein BLAST With
- NCBI/
- ExPASy/
- Uniprot
MHSTTPISSLFSFTSPAVKRLLGWKQGDEEEKWAEKAVDSLVKKLKKKKGAMDELERALSCPGQPSKCVTIPRSLDGRLQVSHRKGLPHVIYCRVWRWPDLQSHHELKPLECCEFPFGSKQKEVCINPYHYRRVETPVLPPVLVPRHSEYNPQLSLLAKFRSASLHSEPLMPHNATYPDSFQQPPCSALPPSPSHAFSQSPCTASYPHSPGSPSEPESPYQHSVDTPPLPYHATEASETQSGQPVDATADRHVVLSIPNGDFRPVCYEEPQHWCSVAYYELNNRVGETFQASSRSVLIDGFTDPSNNRNRFCLGLLSNVNRNSTIENTRRHIGKGVHLYYVGGEVYAECVSDSSIFVQSRNCNYQHGFHPATVCKIPSGCSLKVFNNQLFAQLLAQSVHHGFEVVYELTKMCTIRMSFVKGWGAEYHRQDVTSTPCWIEIHLHGPLQWLDKVLTQMGSPHNPISSVS
Predictions
Score>80(red) has high confidence and is suggested to be used for WB detection. *The prediction model is mainly based on the alignment of immunogen sequences, the results are for reference only, not as the basis of quality assurance.
High(score>80) Medium(80>score>50) Low(score<50) No confidence
PTMs - Q15797/Q99717/O15198 As Substrate
| Site | PTM Type | Enzyme | Source |
|---|---|---|---|
| Ubiquitination | Uniprot | ||
| S11 | Phosphorylation | Uniprot | |
| K21 | Ubiquitination | Uniprot | |
| K32 | Ubiquitination | Uniprot | |
| S78 | Phosphorylation | Uniprot | |
| K81 | Ubiquitination | Uniprot | |
| Y88 | Phosphorylation | Uniprot | |
| K116 | Sumoylation | Uniprot | |
| K116 | Ubiquitination | Uniprot | |
| K118 | Sumoylation | Uniprot | |
| K118 | Ubiquitination | Uniprot | |
| S132 | Phosphorylation | Uniprot | |
| S144 | Phosphorylation | Uniprot | |
| Y146 | Phosphorylation | Uniprot | |
| S187 | Phosphorylation | P50750 (CDK9) , P49336 (CDK8) , P28482 (MAPK1) | Uniprot |
| S195 | Phosphorylation | P49336 (CDK8) , P50750 (CDK9) , P28482 (MAPK1) | Uniprot |
| T202 | Phosphorylation | P49841 (GSK3B) | Uniprot |
| S206 | Phosphorylation | P50750 (CDK9) , P28482 (MAPK1) , P49336 (CDK8) , P50613 (CDK7) | Uniprot |
| S210 | Phosphorylation | Uniprot | |
| S214 | Phosphorylation | P28482 (MAPK1) , P50750 (CDK9) , P49336 (CDK8) | Uniprot |
| S239 | Phosphorylation | Q13315 (ATM) | Uniprot |
| K269 | Ubiquitination | Uniprot | |
| S315 | Phosphorylation | Uniprot | |
| T322 | Phosphorylation | Q8N4C8 (MINK1) , Q9UKE5 (TNIK) | Uniprot |
| K418 | Ubiquitination | Uniprot | |
| S456 | Phosphorylation | Uniprot | |
| S462 | Phosphorylation | O00238 (BMPR1B) | Uniprot |
| S463 | Phosphorylation | Q05655 (PRKCD) , P36894 (BMPR1A) , O00238 (BMPR1B) | Uniprot |
| S465 | Phosphorylation | Q05655 (PRKCD) , O00238 (BMPR1B) , P36894 (BMPR1A) | Uniprot |
| Site | PTM Type | Enzyme | Source |
|---|---|---|---|
| T2 | Acetylation | Uniprot | |
| S12 | Phosphorylation | Uniprot | |
| K22 | Ubiquitination | Uniprot | |
| K33 | Ubiquitination | Uniprot | |
| S57 | Phosphorylation | Uniprot | |
| S58 | Phosphorylation | Uniprot | |
| S63 | Phosphorylation | Uniprot | |
| K64 | Ubiquitination | Uniprot | |
| S79 | Phosphorylation | Uniprot | |
| K82 | Ubiquitination | Uniprot | |
| Y89 | Phosphorylation | Uniprot | |
| K117 | Sumoylation | Uniprot | |
| K119 | Sumoylation | Uniprot | |
| K119 | Ubiquitination | Uniprot | |
| Y128 | Phosphorylation | Uniprot | |
| S133 | Phosphorylation | Uniprot | |
| S152 | Phosphorylation | Uniprot | |
| S188 | Phosphorylation | Uniprot | |
| K306 | Ubiquitination | Uniprot | |
| S315 | Phosphorylation | Uniprot | |
| K418 | Ubiquitination | Uniprot | |
| S462 | Phosphorylation | Uniprot | |
| S463 | Phosphorylation | Uniprot | |
| S465 | Phosphorylation | Uniprot |
| Site | PTM Type | Enzyme | Source |
|---|---|---|---|
| K25 | Ubiquitination | Uniprot | |
| S40 | Phosphorylation | Uniprot | |
| S82 | Phosphorylation | Uniprot | |
| K85 | Ubiquitination | Uniprot | |
| Y92 | Phosphorylation | Uniprot | |
| T136 | Phosphorylation | Q16539 (MAPK14) | Uniprot |
| K159 | Ubiquitination | Uniprot | |
| S317 | Phosphorylation | Uniprot | |
| K420 | Ubiquitination | Uniprot | |
| S458 | Phosphorylation | Uniprot | |
| S464 | Phosphorylation | Uniprot | |
| S465 | Phosphorylation | Uniprot | |
| S467 | Phosphorylation | Uniprot |
Research Backgrounds
Transcriptional modulator activated by BMP (bone morphogenetic proteins) type 1 receptor kinase. SMAD1 is a receptor-regulated SMAD (R-SMAD). SMAD1/OAZ1/PSMB4 complex mediates the degradation of the CREBBP/EP300 repressor SNIP1. May act synergistically with SMAD4 and YY1 in bone morphogenetic protein (BMP)-mediated cardiac-specific gene expression.
Phosphorylation of the C-terminal SVS motif by BMP type 1 receptor kinase activates SMAD1 by promoting dissociation from the receptor and trimerization with SMAD4.
Ubiquitinated by SMAD-specific E3 ubiquitin ligase SMURF1, leading to its degradation. Monoubiquitinated, leading to prevent DNA-binding. Deubiquitination by USP15 alleviates inhibition and promotes activation of TGF-beta target genes. Dephosphorylation, probably by PPM1A, induces its export from the nucleus to the cytoplasm (By similarity).
Cytoplasm. Nucleus.
Note: Cytoplasmic in the absence of ligand. Migrates to the nucleus when complexed with SMAD4 (PubMed:15647271). Co-localizes with LEMD3 at the nucleus inner membrane (PubMed:15647271). Exported from the nucleus to the cytoplasm when dephosphorylated (By similarity).
Ubiquitous. Highest expression seen in the heart and skeletal muscle.
Found in a complex with SMAD4 and YY1. Interacts with HGS, NANOG and ZCCHC12 (By similarity). Upon C-terminus phosphorylation: forms trimers with another SMAD1 and the co-SMAD SMAD4. Interacts with PEBP2-alpha subunit, CREB-binding protein (CBP), p300, SMURF1, SMURF2, USP15 and HOXC8. Associates with ZNF423 or ZNF521 in response to BMP2 leading to activate transcription of BMP target genes. Interacts with SKOR1. Interacts (via MH2 domain) with LEMD3. Binding to LEMD3 results in at least a partial reduction of receptor-mediated phosphorylation. Forms a ternary complex with PSMB4 and OAZ1 before PSMB4 is incorporated into the 20S proteasome. Found in a macromolecular complex with FAM83G. Interacts (via MH2 domain) with FAM83G (via MH2 domain); in a SMAD4-independent manner. Interacts with ZC3H3 (By similarity). Interacts with TMEM119 (By similarity). Interacts (via MH1 and MH2 domains) with ZNF8 (By similarity). Interacts with RANBP3L; the interaction increases when SMAD1 is not phosphorylated and mediates SMAD1 nuclear export.
The MH2 domain mediates phosphorylation-dependent trimerization through L3 loop binding of phosphoserines in the adjacent subunit.
Belongs to the dwarfin/SMAD family.
Transcriptional modulator activated by BMP (bone morphogenetic proteins) type 1 receptor kinase. SMAD5 is a receptor-regulated SMAD (R-SMAD).
Phosphorylated on serine by BMP (bone morphogenetic proteins) type 1 receptor kinase.
Ubiquitin-mediated proteolysis by SMAD-specific E3 ubiquitin ligase SMURF1.
Cytoplasm. Nucleus.
Note: Cytoplasmic in the absence of ligand. Migrates to the nucleus when complexed with SMAD4.
Ubiquitous.
May form trimers with the co-SMAD SMAD4. Interacts with PEBP2-alpha subunit and SMURF1. Interacts with SUV39H1 and SUV39H2. Interacts (via MH2 domain) with LEMD3. Interacts with WWP1. Interacts with TMEM119 (By similarity). Interacts with ZNF8. Interacts with RANBP3L.
Belongs to the dwarfin/SMAD family.
Transcriptional modulator activated by BMP (bone morphogenetic proteins) type 1 receptor kinase. SMAD9 is a receptor-regulated SMAD (R-SMAD).
Phosphorylated on serine by BMP (bone morphogenetic proteins) type 1 receptor kinase.
Cytoplasm. Nucleus.
Note: In the cytoplasm in the absence of ligand. Migration to the nucleus when complexed with SMAD4 (By similarity).
Expressed in heart, brain, placenta, lung, skeletal muscle, prostate, testis, ovary and small intestine. Also expressed in fetal brain, lung and kidney.
Interaction with the co-SMAD SMAD4. Interacts with PEBP2-alpha subunit. Interacts with RANBP3L.
Belongs to the dwarfin/SMAD family.
Research Fields
· Cellular Processes > Cellular community - eukaryotes > Signaling pathways regulating pluripotency of stem cells. (View pathway)
· Environmental Information Processing > Signal transduction > TGF-beta signaling pathway. (View pathway)
· Environmental Information Processing > Signal transduction > Hippo signaling pathway. (View pathway)
· Human Diseases > Cancers: Overview > Transcriptional misregulation in cancer.
References
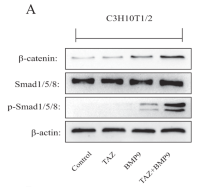
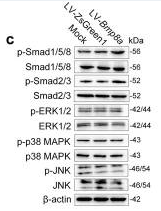
Application: WB Species: rat Sample: BMSCs
Application: WB Species: Mouse Sample: MSCs and MMCs
Application: WB Species: Mouse Sample: 3T3-L1 cells
Application: WB Species: Human Sample: BMMSCs
Application: WB Species: Rat Sample:
Restrictive clause
Affinity Biosciences tests all products strictly. Citations are provided as a resource for additional applications that have not been validated by Affinity Biosciences. Please choose the appropriate format for each application and consult Materials and Methods sections for additional details about the use of any product in these publications.
For Research Use Only.
Not for use in diagnostic or therapeutic procedures. Not for resale. Not for distribution without written consent. Affinity Biosciences will not be held responsible for patent infringement or other violations that may occur with the use of our products. Affinity Biosciences, Affinity Biosciences Logo and all other trademarks are the property of Affinity Biosciences LTD.